Haemophilia Testing
Haemophilia is an inherited bleeding disorder in which blood clotting is impaired
Proteins called clotting factors work with platelets to stop bleeding at the site of an injury. People with haemophilia produce lower amounts of either Factor VIII or Factor IX than those without the condition.
This means the person tends to bleed for a longer time after an injury, and they are more susceptible to internal bleeding.
This bleeding can be fatal if it occurs within a vital organ such as the brain.
Haemophilia tends to occur in males, since the gene can be passed from mother to son.
Types of Haemophilia
There are two main types of haemophilia, type A and type B.
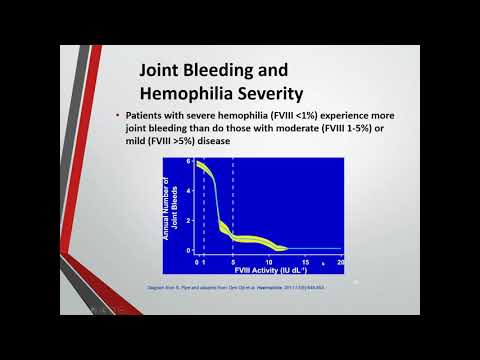
In haemophilia A, there is a lack of clotting factor VIII. This accounts for about 80% of cases. About 70% of people with haemophilia A have the severe form. In haemophilia B, also known as “Christmas disease,” the person lacks clotting factor IX. Haemophilia occurs in around 1 in every 20,000 males born worldwide.
Both A and B can be mild, moderate, or severe, depending on the amount of clotting factor that is in the blood. From 5 to 40 percent of normal clotting factor is considered mild, 1 to 5 percent is moderate, and less than 1 percent is severe.

von Willebrand Disease
von Willebrand disease (vWD) is another genetic bleeding disorder in which patients are prone to frequent bleeding such as nosebleeds, bleeding gums, and excessive menstrual periods.
Unlike haemophilia, vWD affects men and women equally. Like haemophilia, the severity of vWD depends upon the level of the blood protein. The lower the level of protein in the blood, the more severe is the bleeding.
CRYOcheck™ Products
Precision BioLogic's integrated line of high-quality cryocheck™ brand of frozen controls, calibrators and reagents has been specially developed for use in clinical coagulation laboratories.
Factor VIII Testing
Factor VIII is key in the intrinsic clotting cascade.
Testing for factor VIII activity is used to diagnose or monitor the treatment of hemophilia A .
It may also be done to help identify the reason for an abnormal result on other clotting tests (such as prothrombin time [PT] or partial thromboplastin time [PTT]), or when a child has a family member with a bleeding disorder.
For laboratories wishing to accurately quantify Factor VIII activity, we provide the ready-to-use CRYOcheck™ chromogenic
Factor VIII assay.

Factor VIII Inhibitor Testing
In some patients, their immune system doesn’t recognise the factor concentrates (as naturally they produce very little, or no factor VIII) and responds to it as if it were a foreign protein. In these patients, their immune system begins to produce neutralising allo-antibodies, known as inhibitors, against the factor VIII concentrates. The inhibitors bind to the functional epitopes on the infused factor VIII concentrates, affecting the function and rendering the factor concentrates treatment ineffective.
The CRYOcheck™ Factor VIII Inhibitor Kit is able to detect these.

Factor IX Testing
Haemophilia B is a genetic disorder caused by missing or defective factor IX clotting protein. Although it is passed down from parents to children, about 1/3 of cases are caused by a spontaneous mutation, a change in a gene.
The CRYOcheck™ range now includes the chromogenic
Factor IX assay. for use as an aid in the management of haemophilia B in individuals aged two years or older.
Factor Deficient Substrates
CRYOcheck™ Factor Deficient Plasmas are frozen, platelet-poor plasmas which are immunodepleted and assayed at less than
1% for the specific coagulation factor by functional and
antigenic methods.
The frozen format eliminates reconstitution errors and offers products that are ready to use in minutes. All reagents have a long shelf life of three years from date of manufacture.
They may be used in conjunction with the CRYOcheck Gold Standard Family of Assayed Plasmas, including Normal Reference Plasma, Reference Control Normal and Abnormal Reference Controls.

Enhance Your APTT Testing - no more issues with access to congenital FVIII deficient plasma
If you are running the one-stage activated partial thromboplastin time (APTT) method to help manage your haemophilia A patients, the new CRYOcheck™ Factor VIII deficient plasma with Von Willebrand Factor (VWF), could improve your assay performance.
It is currently the only commercially available manufactured factor VIII deficient plasma that compares to using congenital factor VIII deficient plasma. Since access to congenital FVIII deficient plasma is limited and has high donor variability and small pool sizes, this newly available product provides enhanced convenience and increased confidence in testing results.
Literature
-

CRYOcheck™ Chromogenic Factor VIII Assay
Product Brochure
24/12/20 Download: 6.47KB -

CRYOcheck™ Factor VIII Inhibitor Kit
Product Brochure
24/12/20 Download: 1.93MB -

CRYOcheck™ Chromogenic Factor IX Assay
Product Brochure
07/07/22 Download: 14.13KB -

Validation of a New Kit for the Quantitative Determination of Chromogenic Factor IX Activity.
Quinton et al, Presented at ISTH 202107/07/22 Download: 17.09KB
Publications
-
The Impact of Inactive FVIII Antigen in Factor VIII Deficient Plasma on the Measurement of FVIII Inhibitors
-
Comparison of a Chromogenic vs. APTT-based Factor VIII Activity Assay in the Recovery of a Pegylated Factor VIII Replacement Therapy in Plasma Samples
-
Validation of a New Kit for the Quantitative Determination of Chromogenic Factor IX Activity
-
Laboratory Validation of CRYOcheck™ Chromogenic Factor VIII Across Multiple Coagulation Analyzers
-
Performance of CRYOcheck™ Chromogenic Factor VIII in the Recovery of Factor VIII Replacement Therapies
-
A Standardized Kit for a Chromogenic Modified Nijmegen-Bethesda Assay Repeatability, Reproducibility, and Analytical Sensitivity
-
Emicizumab Impact on Factor VIII Inhibitor Determination in Plasma Samples from Persons with Hemophilia A (PwHA) Using a New Kit for Modified Nijmegen-Bethesda Assay (MNBA)
-
Agreement Between a Chromogenic Modified NijmegenBethesda Assay and a Qualitative ELISA test in Detection of Factor VIII Inhibitors in Plasma from Persons with Hemophilia A (PwHA)
-
Performance of a New Kit for a Modified Nijmegen-Bethesda Assay: Comparison of a Chromogenic Versus a Clot-based Factor VIII Inhibitor Assay in Plasma from Persons with Hemophilia A (PwHA)