This product is for Professional Use only and not for sale to the general public for home use.
Please log in with a business account to purchase.

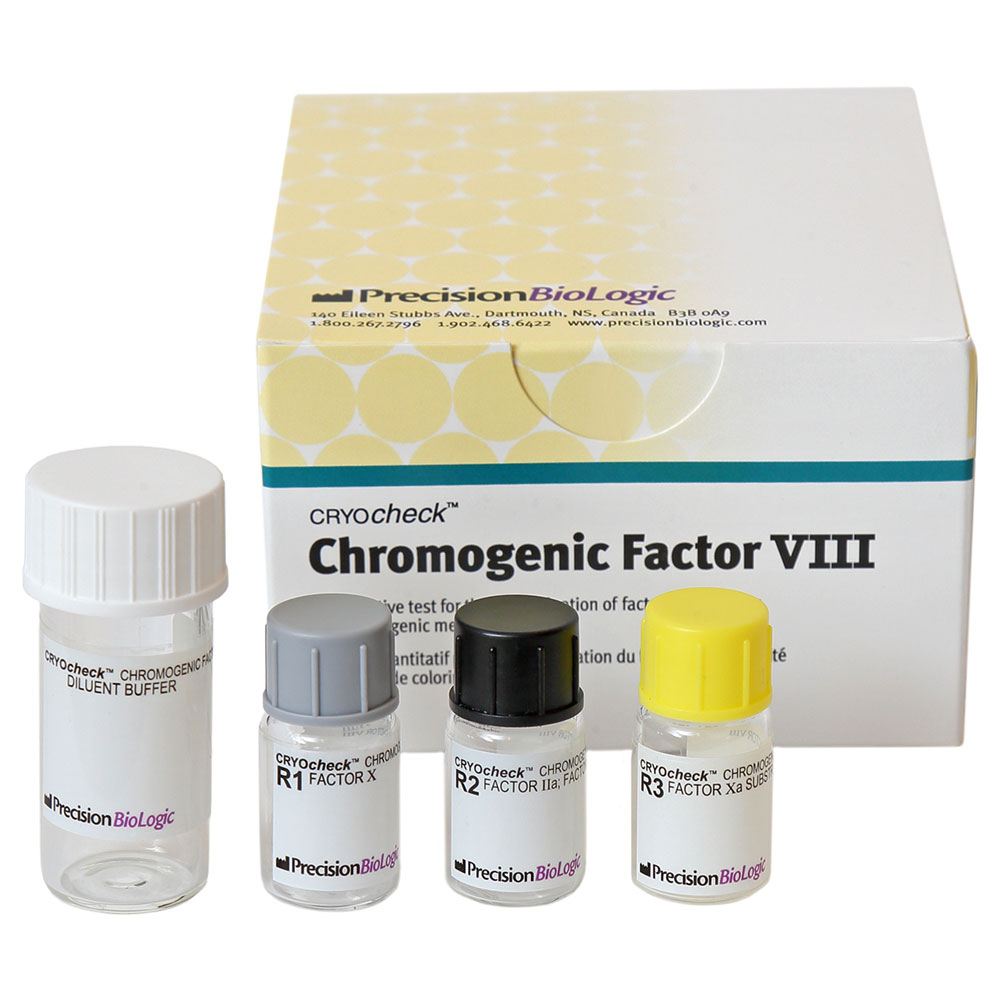
CRYOcheck™ Chromogenic Factor VIII Assay for the determination of factor VIII activity in human plasma to aid in the management of haemophilia A
Product code: CCCF08
Pack Size: Kit
CE Verified: CE IVD
Contact us
This is not a regular stock item and therefore not subject to our standard delivery schedule. Please contact us on +44 (0) 2380 483000 for advice on expected delivery date.
Product Information
CRYOcheck Chromogenic Factor VIII is for clinical laboratory use in the quantitative determination of factor VIII activity in 3.2% citrated human plasma. It is intended to be used in identifying factor VIII deficiency and as an aid in the management of haemophilia A in individuals aged 2 years and older. For in vitro diagnostic use.
Factor VIII is a critical component in the normal blood clot-forming process; upon activation of the coagulation cascade, it binds to factors IXa and X (FIXa and FX) to enable activation of FX and subsequent downstream thrombin activation, which leads to cleavage of fibrinogen and formation of a polymerized fibrin clot. Haemophilia A is an inherited bleeding disorder caused by a decrease or functional deficiency in factor VIII (FVIII), which leads to a lifelong bleeding tendency. The standard treatment for patients with haemophilia A without inhibitors is intravenous (IV) FVIII replacement therapy with recombinant FVIII (rFVIII) or plasma-derived FVIII (pdFVIII) concentrates. CRYOcheck Chromogenic Factor VIII is used for determination of FVIII activity. Unlike, clot-based FVIII activity tests, the chromogenic method offers the advantage of being less prone to interference from lipids or traces of heparin in samples.
CRYOcheck Chromogenic Factor VIII is a two-stage assay for use with automated coagulometers. In the first stage of the chromogenic assay, test plasma (containing an unknown amount of FVIII) is added to a reaction mixture comprised of calcium, phospholipids, purified human thrombin and FIXa, and FX of bovine origin. This mixture swiftly activates FVIII to FVIIIa, which works in concert with FIXa to activate FX. When the reaction is stopped, FXa production is assumed to be proportional to the amount of functional FVIII present in the sample. The second stage of the assay is to measure FXa through cleavage of a FXa-specific peptide nitroanilide substrate. The product (p-nitroaniline) produces a yellow colour that can be measured spectrophotometrically by absorbance at 405 nm. The colour produced is directly proportional to the amount of functional FVIII present in the sample based on a standard curve.
Factor VIII is a critical component in the normal blood clot-forming process; upon activation of the coagulation cascade, it binds to factors IXa and X (FIXa and FX) to enable activation of FX and subsequent downstream thrombin activation, which leads to cleavage of fibrinogen and formation of a polymerized fibrin clot. Haemophilia A is an inherited bleeding disorder caused by a decrease or functional deficiency in factor VIII (FVIII), which leads to a lifelong bleeding tendency. The standard treatment for patients with haemophilia A without inhibitors is intravenous (IV) FVIII replacement therapy with recombinant FVIII (rFVIII) or plasma-derived FVIII (pdFVIII) concentrates. CRYOcheck Chromogenic Factor VIII is used for determination of FVIII activity. Unlike, clot-based FVIII activity tests, the chromogenic method offers the advantage of being less prone to interference from lipids or traces of heparin in samples.
CRYOcheck Chromogenic Factor VIII is a two-stage assay for use with automated coagulometers. In the first stage of the chromogenic assay, test plasma (containing an unknown amount of FVIII) is added to a reaction mixture comprised of calcium, phospholipids, purified human thrombin and FIXa, and FX of bovine origin. This mixture swiftly activates FVIII to FVIIIa, which works in concert with FIXa to activate FX. When the reaction is stopped, FXa production is assumed to be proportional to the amount of functional FVIII present in the sample. The second stage of the assay is to measure FXa through cleavage of a FXa-specific peptide nitroanilide substrate. The product (p-nitroaniline) produces a yellow colour that can be measured spectrophotometrically by absorbance at 405 nm. The colour produced is directly proportional to the amount of functional FVIII present in the sample based on a standard curve.
Specification
- Brand Precision Biologic
- Sterile N
- CE Certified CE IVD
- Warranty Period Standard Terms Apply
- Is product classified as dangerous goods? No
- Does the product contain latex? Unspecified
- Application Chromogenic Factor VIII Testing
- Contents Reagent 1: Bovine FX and a fibrin polymerisation inhibitor, plus activators and stabilisers 1 x 1.25 mL
Reagent 2: Human FIIa, human FIXa, calcium chloride and phospholipids 1 x 1.25mL
Reagent 3: FXa substrate containing EDTA and a thrombin inhibitor 1 x 1.25mL
Diluent Buffer: Tris buffer solution containing 1% BSA and a heparin antagonist 1 x 7.0 mL - Shelf Life 12 months
Storage Details
- Pack Description Kit
- Shipping Condition Dry Ice
- Storage Condition -80
- Pack Length (m) 0.3
- Pack Width (m) 0.3
- Pack Height (m) 0.3
- Pack Weight (kg) 0.00