Pyrogen Testing
Pyrogens are foreign bodies or materials in the body that induce various biological reactions when even a small amount enters the bloodstream, examples are endotoxin, peptidoglycan (PG) and (1->3)-β-D-glucan.
They are fever-inducing substances usually derived from microorganisms.
Products contaminated with pyrogens can cause severe consequences as they elicit a strong immune response that can escalate rapidly. Pyrogen testing is essential for the manufacturing of pharmaceutical products, biologicals for injection, medical devices, and media for tissue cultures.
KCA is a quantitative test that uses synthetic chromogenic substrate cleavage to detect the activation of the LAL reagent induced by endotoxin.
GCA is a qualitative test to detect endotoxin by the presence of gel formation. KTA is a quantitative test that uses the change in gel turbidity to detect the activation of the LAL reagent induced by endotoxin.
KTA is a quantitative test that uses the change in gel turbidity to detect the activation of the LAL reagent induced by endotoxin.
Quantitative determination of peptidoglycan (PG) and (1->3)-β-D-glucan in parental solutions and active pharmaceutical.
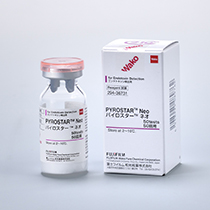
PYROSTAR™ Neo is a genetically engineered alternative for endotoxin detection for pharmaceutical and medical equipment manufacturing.
Accessories for use with any of the available Pyrogen Testing kits, including pyrogen and endotoxin free test tubes, pipette tips and microplates.
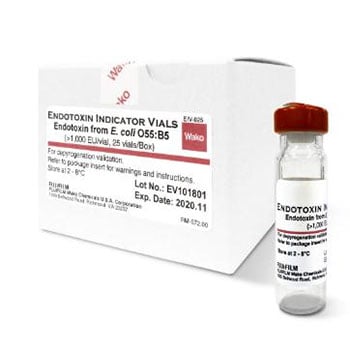
Reagents for use with any of the available Pyrogen Testing kits, including the ES buffer for specific endotoxin testing, endotoxin free reagent water, endotoxin indicators, endotoxin extracting solution and curdlan.