This product is for Professional Use only and not for sale to the general public for home use.
Please log in with a business account to purchase.

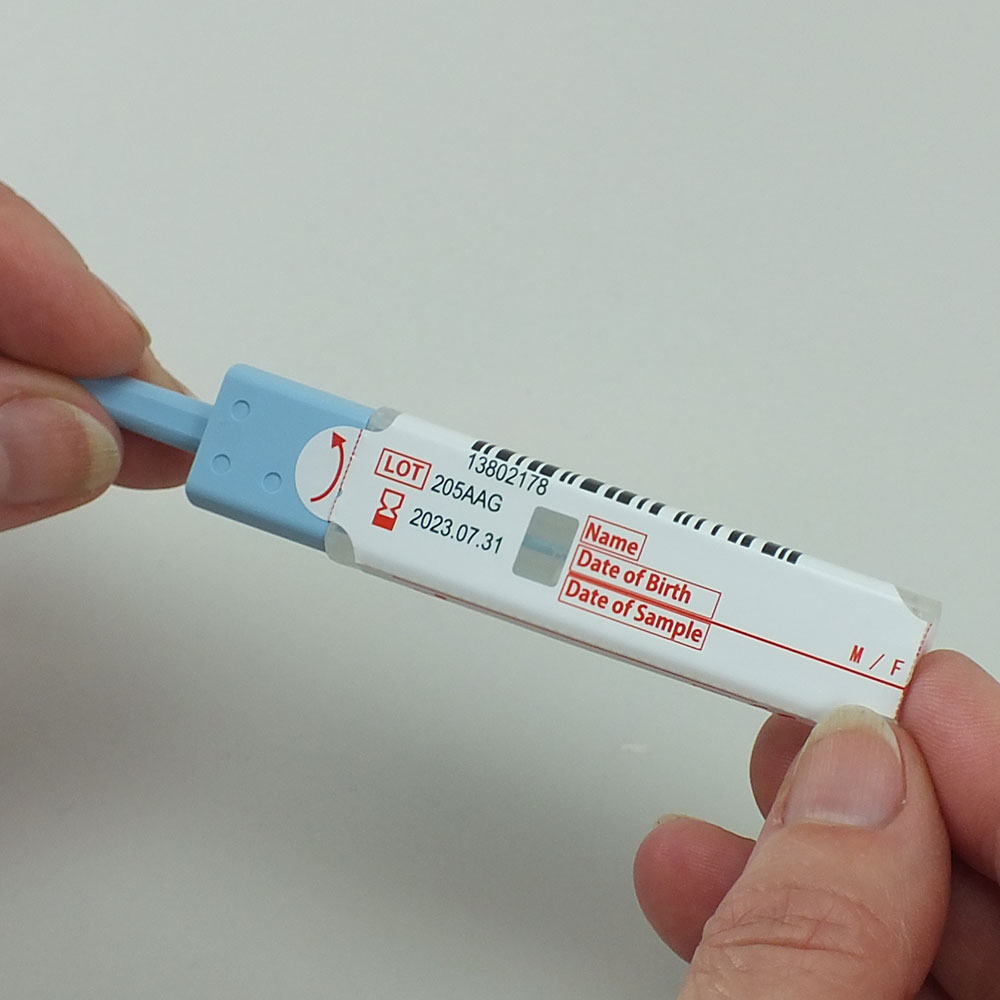
Faecal immunochemical test sample Collection Picker. To collect, preserve, and test samples for analysis on the HM-JACKarc analyser. Pack of 200.
Product code: 063631
Pack Size: 200
CE Verified: CE IVD
Product Information
The EXTEL HEMO-AUTO MC Collection Picker is a sample collection device which facilitates sample collection and preservation for faecal immunochemical testing.
Background:
Blood in faeces could be a symptom of a range of gastroenterological complaints: colorectal cancer (CRC), inflammatory bowel disease (IBD), and diverticular disease, for example. The use of a marker such as blood in the stool has been used in CRC screening for over 10-years, originally with a guaiac based, qualitative technology, faecal occult blood (gFOB). Faecal immunochemical tests (FIT) replaced the use of guaiac-based tests as they have a greater sensitivity and specificity.
Clinical Evidence:
The FIT collection device, when used in conjunction with the dedicated bench-top analyser, the HM-JACKarc, has a sensitivity of 100%, and a specificity of 76.6% (NICE, 2017) for detecting colorectal cancer. The test has a 100% negative predictive value (NPV, true negative value) which gives confidence in the fact that a negative FIT means the chances of blood in the stool is very low. The analyser has a limit of detection (LoD) of 1.25 µg Hb per g faeces and a limit of quantitation of 7 µg Hb / g faeces, giving you confidence in results below the recommended cut-off of 10 µg Hb / g faeces (NICE, 2017).
Collecting the Sample:
The collection device is designed with two small dimples in the end of the sampling stick to allow the patient to accurately sample 2 mg of faeces with no laboratory experience or measuring equipment. The septum in the sample collection device scrapes off excess faecal matter, ensuring the 2 ml of buffer solution is accurately dosed with 2 mg of stool. This offers a hygienic sampling method, which is easy to use, and provides good quality samples for laboratory analysis. The device contains a biocide to minimise the risk of haemoglobin degradation by the microbiome in the stool, and the device is tested to a 95 kPa pressure differential: making the tube strong and leak-proof.
Laboratory Analysis:
Once the sample is collected, the collection device is stable for 14-days at room temperature, and 120-days at 2 - 8 C. The sample is pre-diluted (in a 1:1 ratio) and is ready to be loaded directly onto the analyser. The analyser reports results in ng Hb per ml of buffer; since the HM-JACKarc uses a 1:1 dilution ratio, this can be directly converted (1:1) to µg Hb per gram for result reporting. The analyser has a throughput of 200 sample per hour. Time to first result is 5.6 minutes, with subsequent results every 18 seconds. The sample does not require any pre-analytical processing (dilution / filtration etc.) prior to loading.
Product Specification:
The collection devices come in a pack of 200. The sampling device is packaged into a small green sample bag, along with a generic, multi-language sampling instruction leaflet for the patient. Also available in a pack of 400, see code 063632.
NICE (2017) Diagnostic Guidance DG30: Quantitative faecal immunochemical tests to guide referral for colorectal cancer in primary care. [Online]. Available from: www.nice.org.uk/guidance/dg30
Background:
Blood in faeces could be a symptom of a range of gastroenterological complaints: colorectal cancer (CRC), inflammatory bowel disease (IBD), and diverticular disease, for example. The use of a marker such as blood in the stool has been used in CRC screening for over 10-years, originally with a guaiac based, qualitative technology, faecal occult blood (gFOB). Faecal immunochemical tests (FIT) replaced the use of guaiac-based tests as they have a greater sensitivity and specificity.
Clinical Evidence:
The FIT collection device, when used in conjunction with the dedicated bench-top analyser, the HM-JACKarc, has a sensitivity of 100%, and a specificity of 76.6% (NICE, 2017) for detecting colorectal cancer. The test has a 100% negative predictive value (NPV, true negative value) which gives confidence in the fact that a negative FIT means the chances of blood in the stool is very low. The analyser has a limit of detection (LoD) of 1.25 µg Hb per g faeces and a limit of quantitation of 7 µg Hb / g faeces, giving you confidence in results below the recommended cut-off of 10 µg Hb / g faeces (NICE, 2017).
Collecting the Sample:
The collection device is designed with two small dimples in the end of the sampling stick to allow the patient to accurately sample 2 mg of faeces with no laboratory experience or measuring equipment. The septum in the sample collection device scrapes off excess faecal matter, ensuring the 2 ml of buffer solution is accurately dosed with 2 mg of stool. This offers a hygienic sampling method, which is easy to use, and provides good quality samples for laboratory analysis. The device contains a biocide to minimise the risk of haemoglobin degradation by the microbiome in the stool, and the device is tested to a 95 kPa pressure differential: making the tube strong and leak-proof.
Laboratory Analysis:
Once the sample is collected, the collection device is stable for 14-days at room temperature, and 120-days at 2 - 8 C. The sample is pre-diluted (in a 1:1 ratio) and is ready to be loaded directly onto the analyser. The analyser reports results in ng Hb per ml of buffer; since the HM-JACKarc uses a 1:1 dilution ratio, this can be directly converted (1:1) to µg Hb per gram for result reporting. The analyser has a throughput of 200 sample per hour. Time to first result is 5.6 minutes, with subsequent results every 18 seconds. The sample does not require any pre-analytical processing (dilution / filtration etc.) prior to loading.
Product Specification:
The collection devices come in a pack of 200. The sampling device is packaged into a small green sample bag, along with a generic, multi-language sampling instruction leaflet for the patient. Also available in a pack of 400, see code 063632.
NICE (2017) Diagnostic Guidance DG30: Quantitative faecal immunochemical tests to guide referral for colorectal cancer in primary care. [Online]. Available from: www.nice.org.uk/guidance/dg30
Specification
- Brand Minaris Medical
- Sterile N
- CE Certified CE IVD
- Warranty Period Standard Terms Apply
- Is product classified as dangerous goods? No
- Does the product contain latex? Unspecified
Storage Details
- Pack Description 200
- Shipping Condition Ambient
- Storage Condition Ambient
- Pack Length (m) 0.37
- Pack Width (m) 0.28
- Pack Height (m) 0.18
- Pack Weight (kg) 5.00